Overview
The pE-4000 sets the standard as the universal Illumination System for fluorescence microscopy. The system has 16 selectable LED wavelengths across four channels that can be finely controlled and matched to the filters and fluorophores of almost any microscope, making it the broadest and most versatile illumination system available.
The CoolLED pE-4000 benefits from our award winning sustainable Green technology and delivers enhanced irradiance at the sample plane with a significant reduction in energy consumption, and is supplied with a 36 month warranty.
The pE-4000 is a powerful, flexible, controllable system for advanced research applications.
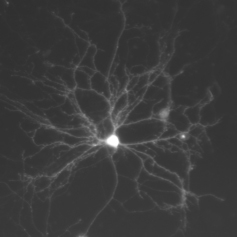
“Striking, bright fluorescence images with a strong signal to noise ratio even when using a low magnification objective”
Graham Wright, Institute of Medical Biology, A*STAR Singapore
Advanced control for cutting-edge research
Multiple levels of control are possible, from simple to advanced. A dual mode control pod gives immediate manual control in either White or Advanced modes, where individual LED channels can be individually switched on/off or irradiance modulated.
Alternatively, the USB interface and integration into major microscope software platforms allows complex software control.
TTL control enables capturing high-speed events (<1 ms). Since there is no shutter or filter wheel, without these moving parts, this reduced latency means speed is limited only by the camera. An expansion box is also available for added functionality with multiple BNC connections for remote analogue and TTL control.
For optogenetics applications, alongside the ability to include excitation filters to tune the wavelength to the opsin’s maximum activation peak, an internal function generator controls pulse timing and waveforms.
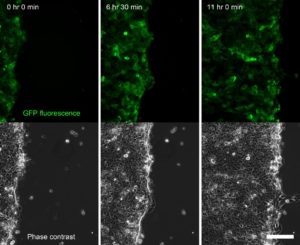
Improving cell viability
With the pE-4000, it is now possible to prevent artefacts and allow live samples to thrive in experiments >100 hours. Fine control of illumination timing and irradiance with TTL and the function generator minimises exposure, avoiding photobleaching and phototoxicity.
“When you can only control the intensity of ‘white’ light (rather than individual channels), the level of photobleaching can be high. With the pE-4000, we can control the excitation of the individual channels. It is possible to optimise the excitation irradiance according to the labelling, greatly reducing photobleaching and phototoxicity in a live experiment.”
Dr Yan Gu, University of Sussex
“Suddenly, we were able to offer users uninterrupted extended live cell experiments of 100+ hours, without worrying about brightness fluctuations, lamps burning, room heating, etc. Also, users have reported markedly reduced bleaching and phototoxicity in their samples, both from the prokaryotic and the eukaryotic research fields.”
Dr Jens Eriksson, Oslo University Hospital
Matching diverse fluorophore requirements
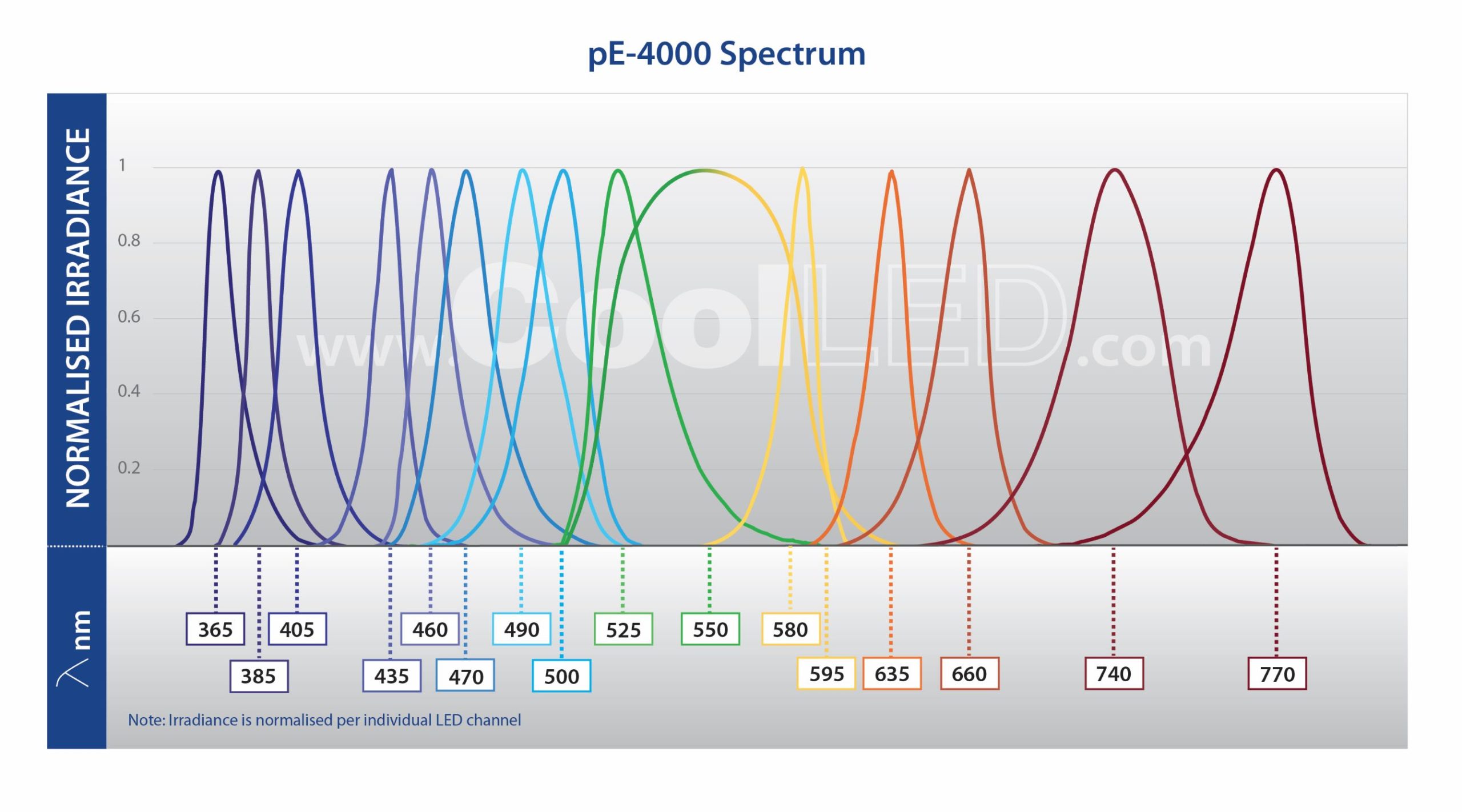
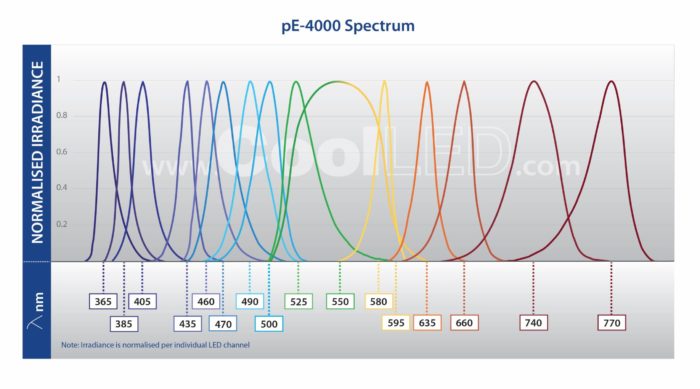
Full filter compatibility and 256 wavelength combinations from 16 installed LEDs (365 nm -770 nm) covers all available fluorophores, which is ideal for multi-user facilities or evolving experimental needs.
Multi-channel experiments with no pixel shift
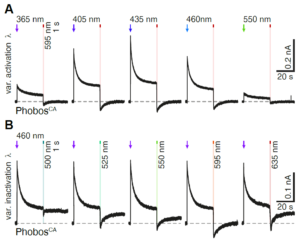
Up to four channels using distinct wavelengths can be captured with no mechanical movement, preventing pixel shift and allowing the direct comparison of data as in the following experiment:
(A) Representative photocurrent traces of a PhobosCA expressing CA1 cell evoked with different activation wavelengths and shutoff with 595 nm light. (B) Photocurrent traces in the same cell evoked with 460 nm light and shutoff with indicated wavelengths (10 mW/mm2).1
Increased power efficiency
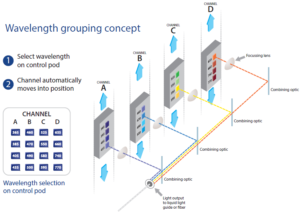
The pE-4000 includes 16 LEDs grouped into four individually controllable channels, and this unique Wavelength Grouping Concept makes it possible to deliver more power in an efficient four-channel system. As the first company to introduce LED microscope lighting for fluorescence microscopy, CoolLED has developed a comprehensive understanding of the complexities of configuring and selecting filter sets for experiments using multiple fluorophores. CoolLED’s innovation comes from recognising that all the fluorophores used in multi-band work can have their absorption bands divided into four separate groups across the spectrum due to the restricted bandwidth available.
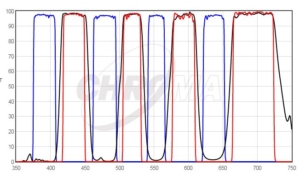
DAPI/FITC/TRITC/Cy5 Quad filter excited by pE-4000 at matched wavelengths shown above.
A choice of 16 LEDs enables the user to select the optimal excitation wavelength for each fluorophore combination and optical filter configuration, which enables maximum performance and flexibility. Our Filter Finder tool provides more information on recommended filter configurations.
Performance
- Broadest LED spectrum available: 365-770 nm, with 16 LEDs
- Four channel wavelength-grouping design matches most commercially available dual, triple, and quad filters
- Removable inline excitation filter holders: no moving parts for fast acquisition
- Internal function generator for electrophysiology and optogenetics applications
- TTL and USB interfaces with major imaging software packages allows incorporation into all experimental parameters
- Analogue inputs for dynamic irradiance control
- Individual control of selected LED wavelengths reduces background
- Rapid switching between LED wavelengths enables capture of high-speed events
- Instant on/off: No shutters required, no warm up or cool down
- Stable and repeatable: reliable and consistent results
- Precise irradiance control in 1% steps (0-100%): no ND filters required
Convenience
- Pre-sets allow lab manager to match white spectrum to existing filter cubes
- Simple to fit, simple to use: no alignment, a once only adjustment
- Wide range of microscope adaptors: fits most microscopes
Plus all the benefits of LED technology.
The system comprises a pE-4000 Light Source, Control Pod, liquid light guide, Universal Collimator and Power Supply.
Control
Manual:
Dual function remote manual control pod for White mode or Advanced mode
Remote:
Via USB for independent on/off and irradiance control of each channel. Triggering speed approximately 10-20 ms
Via 4 TTL inputs for independent on/off control of each channel. Triggering speed <20 µs
Via single TTL for on/off control of manual or software selected channels
Via 4 analogue inputs 0-10 V, 0-300 kHz for dynamic control of irradiance from external analogue signals
Sync Out:
4 TTL outputs for each channel – active high 1 TTL output for any channel – active high
Programmable interface:
4 TTL outputs for on/off control of peripherals (transmitted illuminators, stages etc.) 4 analogue outputs for irradiance control of peripherals (can be programmed to mirror LED irradiance levels for channel control) 0-10 V full scale.
Function Generator:
Internally generated sine, pulse and ramps for each channel programmed via control pod.
Connectivity:
USB (B type) for PC connection. All other TTL and Analogue inputs/outputs via 25-way ‘D-type’ female connector (optional rear mounting pE-Expansion Box available for BNC connectivity).
Imaging Software:
Recognised under common software e.g. Micromanager, MetaMorph, cellSens, NIS Elements, etc. see Imaging Software
Choosing configurations
The pE-4000 can be configured to deliver light to the microscope via liquid light guide or fibre. Liquid light guides can be used in conjunction with CoolLED’s pE-Universal Collimator which can accept a microscope adaptor from CoolLED’s extensive range. Find out more here.
Specification
Power requirements
110-240 V a.c. 50/60 Hz
Power consumption
Standby (i.e. no LEDs on) : Max 7 W
Single wavelength operation : Max 25 W
Dual wavelength operation : Max 44 W
Triple wavelength operation : Max 53 W
Quad wavelength operation : Max 60 W
Dimensions
pE-4000 Light Source: 150 mm(w) x 22 0mm(d) x 260 mm(h) – Weight 3.5 kg
pE-4000 Control pod: 154 mm(w) x 135 mm(d) x 40 mm(h) – Weight 0.95 kg
pE-4000 Power Supply: 164 mm(w) x 64 mm(d) x 35 mm(h) – Weight 0.58 kg
pE-Expansion Box: 151 mm(w) x 18 mm(d) x 95 mm(h) – Weight 0.34 kg
Environment and Safety
- Mercury-free and Laser-free
- Energy Efficient
- Long lifetime
- No bulb replacements
- Reduced risk of eye damage
- Quiet operation
- No special disposal regulations or issues
Warranty
Industry-leading 36 months. Read more here.
All data correct at time of publication
We are often asked about the power, intensity or irradiance of CoolLED Illumination Systems. The answer is not as simple as you might believe from many websites and data sheets, and it can be difficult to compare data from these sources as measuring set ups differ.
Photons are also lost as light travels from the light source to sample plane. The only way to objectively compare light sources is by measuring irradiance at the sample plane. To understand why we use the term irradiance and how to measure and compare light sources with accuracy and precision, download our white paper. Or please contact us if you have further questions.
For performance data, please contact us
The customer service at CoolLED is phenomenal! Justyna was amazing with product feedback. I have NO hesitation recommending CoolLED for their products and service. Simply fantastic!
Rama Gullapalli - Assistant Professor, Department of Pathology, and Chemical & Biological Engineering, University of New Mexico (CoolLED Support)
We bought two pE-4000 Illumination Systems, and they are just great for us - many thanks for this all-purpose product!
Dr Oliver Thorn-Seshold, Thorn-Seshold Group Leader, LMU Munich (pE-4000)
We enjoyed the versatility of the pE-4000: the number of applications of this illumination system is immense! We use multiple units in the lab as light sources for epifluorescence microscopy, for multi-color optogenetic stimulation and for fiber photometry experiments.
The Research Group, Synaptic Wiring and Information Processing, Center for Molecular Neurobiology Hamburg (ZMNH) (pE-4000)
From my perspective the pE-4000 has improved our system in many ways; it is one of the best upgrades we have done recently, and I would never go back.
Dr Christian Rudolph, Principal Investigator/Reader, Brunel University London (pE-4000)
The large range of wavelengths available in the CoolLED pE-4000 and the ability to digitally trigger them with millisecond precision when stimulating neurons allows greater flexibility in choosing the most appropriate opsin for a given experiment and stimulating it at the peak of its excitation spectrum.
Alessandro Galloni, PhD Student, The Rancz Lab, Francis Crick Institute, London (pE-4000)
The CoolLED pE-4000 device performed excellently in our electrophysiological experiments and provided us with a convenient way to investigate the integrative properties of hippocampal neurons in great detail.
Attila Szücs, Eötvös Lóránd University, Hungary (pE-4000)
Our CoolLED pE-4000 is installed on the spinning disk confocal. Our use of the pE-4000, for now, has been mostly limited to specimen finding and focusing. We can certainly already say that it is a very powerful and very flexible system compared to the traditional fluorescent lamp that we've installed on the other microscopes. The fact of not having neutral density filters makes the variation in light intensity much more linear. To date, we have verified that for many samples it is sufficient to use LEDs with a maximum power of 5-10%. We are also very confident about the maximum lifespan of LEDs compared to that of classic fluorescent lamps. The system interface is very user friendly, both through the Nikon NIS software to its controller.
Professor Alex Costa, Professor in Plant Physiology, Univeristà degli Studi di Milano, Italy (pE-4000)
Our precisExcite is working in a fantastic manner for over 10 years. It has already contributed to more than 10 publications from the lab and will continue to do so.
Charles Harata, Associate Professor, University of Iowa (pE-4000)
I have been using a pE-4000 for optogenetic experiments using in vitro slices for just over a year now. The pE-4000 has been fantastic for my needs: the ability to simultaneously use multiple wavelengths of light has allowed me to carry out experiments using two different opsins to study how external inputs converge on hippocampal interneurons. I’m currently setting up my own research group at the University of Exeter, and all of my patch-clamp rigs will have a pE-4000 on them, as they are such versatile devices for optogenetic experiments.
Dr Michael Craig, Research Fellow, University of Exeter Medical School (pE-4000)
Our group is currently attempting to isolate and stain circulating tumour cells. In order to identify these cells we need a minimum of four colour fluorescence imaging, requiring the slide to be scanned using four different LEDs. To stitch our images together after scanning we require a transmission light microscope image. The image co-ordinates of the light microscope image are used to stitch the fluorescence images together. The previous light source (pE-2) allowed only three LEDs to be used together for our purposes. This meant that when we have multiple slides there are two options to get the full four fluorescence scan:1) Change the LED after scanning in Brightfield and three fluorescence channels – this risks damage to the LED itself and is time consuming. It involves un-screwing a panel each time and screws can be damaged or lost relatively easily. It places demand on the ribbon that is connected to the LED and, over time, may damage the ribbon also. We usually scan multiple slides and prefer to use option two, to reduce wear and tear on the light source.2) This option is to scan all slides in Brightfield and three colour fluorescence and then go for a second batch of images in both Brightfield and the final fluorescence channel. This is also time consuming, as setting up each scan in focus takes time. It also requires a lot more downstream processing to line the images up with one another, as the scans are often of two different sizes. With the new pE-4000 light source I was able to scan four slides in Brightfield and four fluorescence channels in three hours. Four slides took six hours to scan on the previous light source, due to double the number of scans and changing the LED. It has also reduced the downstream processing of the images, as they are all taken using the same co-ordinates, in one batch. Our project aims to expand from four colour fluorescence to six or seven channels over the coming years. Using the old light sources this would require a minimum of three but possibly four scans taken from different positions per slide and would take hours per slide. The current light source meets the needs of researches using modern immunofluorescence techniques as most projects have moved from single to a multi-marker approach, in recent years.
Anthony Cooney, PhD Student, Department of Histopathology, Trinity College Dublin (pE-4000)
I have been imaging using the new CoolLED pE-4000 system on our epi-fluorescence microscope. This is an excellent system as it covers a very wide spectrum of wavelengths. I have found it very easy to use. The user interface is much better as the different LEDs can be changed using the handheld control- without switching off and unscrewing the LAMs as had to be done on the old pE-2 system. In addition the LEDs can be changed dynamically while the software to capture the image is open, and also a huge combination of different wavelengths can be used at the same time. I have found that the light source from this system is considerably stronger than from the previous system - I now image at 50% strength, when I used to image at 100%. I anticipate using this imaging system a lot more now that the new LED light source is there.
Alison Reynolds, PhD Research Fellow, UCD Conway Institute, University College Dublin (pE-4000)
I am very impressed with the brightness and performance of the CoolLED pE-4000 in comparison to the mercury or halide light sources for fluorescence. I also really like its neat packaging and it is easy to set up and configure. Absolutely brilliant upgrade for archaic mercury set ups!
Dominic White, Area Sales Manager, Carl Zeiss Microscopy GmbH (pE-4000)
Throwing out the old mercury lamp and exchanging it for the pE-4000 on one of our live cell wide field microscopes has been an astounding success! It allowed us to cheaply breathe new life into old equipment. Suddenly, we were able to offer users uninterrupted extended live cell experiments of 100+ hours, without worrying about brightness fluctuations, lamps burning, room heating, etc. Also, users have reported markedly reduced bleaching and phototoxicity in their samples, both from the prokaryotic and the eukaryotic research fields. As a facility manager I am pleased with the instrument's ease of use, and shallow learning curve for new users. Taken together with reduced overall microscope maintenance, the pE-4000 has already saved me a substantial amount of time in my daily work life, and improved the quality of our services at the same time.
Dr. Jens Eriksson, Manager, Superresolution Microscopy Core Facility, Dep. Clinical Biochemistry, Oslo University Hospital (pE-4000)
I just purchased a pE-4000 to replace an old version of Lumencor Sola SE on a live imaging microscope. After comparing the power level of the systems, I am convinced that the pE-4000 provides stronger illumination at the objective. As the Sola light source can only control intensity of ‘white’ light (rather than individual channels), we have to choose the excitation level according to the dimmest labelling fluorophore. The other channels are in turn exposed to an unnecessary high dose of excitation. The level of photo-bleaching can be high as a result. With the pE-4000, we can control the excitation of the individual channel. It is possible to optimise the excitation intensity according to the labelling, greatly reducing photo-bleaching and photo-toxicity in a live experiment. Due to the difference in the excitation spectrum with the Sola, I was worried about the extra cost of changing the dichroic, excitation and emission filters. The options of four wavelength in each channel (totally four channels) enables me to pick up the correct wavelength according to the old filter/dichroic settings.
Dr. Yan Gu, Manager, Analytical and Quantitative Light Microscopy Facility, Genome Damage and Stability Centre, University of Sussex (pE-4000)
Research Papers
- Widespread prevalence of a methylation-dependent switch to activate an essential DNA damage response in bacteria
- An Improved Workflow for the Quantification of Orthohantavirus Infection Using Automated Imaging and Flow Cytometry
- Photonic neural probe enabled microendoscopes for light-sheet light-field computational fluorescence brain imaging
- Influence of microgravity on spontaneous calcium activity of primary hippocampal neurons grown in microfluidic chips
- Time-lapse mesoscopy of Candida albicans and Staphylococcus aureus dual-species biofilms reveals a structural role for the hyphae of C. albicans in biofilm formation
White Papers
| Light Source | Application | Type | CoolLED LED | Chroma | Semrock | |||
|---|---|---|---|---|---|---|---|---|
| pE-4000 (LLG Fit) | Fluorescence | Single | 365 | 49053 CoolLED DAPI | 49000 ET Dapi | LED-DAPI-B | ||
| pE-4000 (LLG Fit) | Fluorescence | Single | 385 | 49053 CoolLED DAPI | 49028 ET Dapi 395 | LED-DAPI-B | CFW-BP01- Clinical | |
| pE-4000 (LLG Fit) | Fluorescence | Single | 405 | 49053 CoolLED DAPI | 49028 ET Dapi 395 | LED-DAPI-B | ||
| pE-4000 (LLG Fit) | Fluorescence | Single | 435 | 49001 ET eCFP | 49013 ET Teal | LED-CFP-A | LED-mTFP-A | |
| pE-4000 (LLG Fit) | Fluorescence | Single | 460 | 49054 CoolLED GFP | GFP-4050B | GFP-A-Basic | ||
| pE-4000 (LLG Fit) | Fluorescence | Single | 460 | 49002 ET eGFP | 39002 AT eGFP | GFP-4050B | GFP-A-Basic | |
| pE-4000 (LLG Fit) | Fluorescence | Single | 490 | 49303 ET Green#1 | 49003 ET eYFP | YFP-A-BASIC | ||
| pE-4000 (LLG Fit) | Fluorescence | Single | 550 | 49004 ET Cy3 | 49005 ET dsRed | TxRed-4040C | LED-TRITC-A | |
| pE-4000 (LLG Fit) | Fluorescence | Single | 580 | 49055 CoolLED mCherry #1 | 49056 CoolLED mCherry #2 | TxRed-4040C | LED-mCherry-A | |
| pE-4000 (LLG Fit) | Fluorescence | Single | 580 | 49008 ET mCherry | 39010 AT Tex. Red | YFP-2427B | LED-mCherry-A | |
| pE-4000 (LLG Fit) | Fluorescence | Single | 595 | E9015 ET Alexa 633 | SpRed-B | |||
| pE-4000 (LLG Fit) | Fluorescence | Single | 635 | 49006 ET CY5 | 39007 AT Cy5 | Cy5-4040C | ||
| pE-4000 (LLG Fit) | Fluorescence | Single | 660 | 49022 ET Cy5.5 | Cy5.5-C | |||
| pE-4000 (LLG Fit) | Fluorescence | Single | 740 | 49007 ET Cy7 | 49037 ET LICor 800 | IRDYE800-33LP-A | Cy7-B | |
| pE-4000 (LLG Fit) | Fluorescence | Single | 770 | 49030 ET Indocy. Gr. | ICG-B | |||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | (365,385,405), 460, 550 | (365, 385, 405), 460, 580 | 69401 – ET Triple Multi LED | LED-DA/FI/TX-A | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 405, 490, 550, 635 | (365, 385, 405), 460, 580, 635 | 89402 ET DAPI/FITC/TRITC/Cy5 | LED-DA/FI/TR/Cy5-B | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 385, 490, 550 | 385, 460, 550 | 69000 ET DAPI/FITC/TRITC | DA/FI/TR-A | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 385, 490, 580 | 69015 ET DAPI/Green/RedFISH | ||||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 405, 490, 550 | 69010 ET DAPI/FITC/Cy3 | ||||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 405, 490, 550 | 405, 490, 580 | 69002 ET DAPI/FITC/Texas Red | DA/FI/TX-B | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 435, 490, 550 | 69011 ET Aqua/Green/Orange FISH | ||||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 435, 490, 580 | 435, 490, 580 | 69008 ET eCFP/eYFP/mCherry | LED-CFP/YFP/mCherry-A | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 405, 490 | 385, (460, 490) | 59001v2 ET DAPI/Green FISH | DA/FI-A | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 405, 550 | 59002v2 ET DAPI/Orange | ||||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 405, 580 | (460, 490), 580 | 59003 v2 ET DAPI/Red FISH | FITC/TxRed-A | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 435, 490 | 405, 490 | 59017 ET eCFP/eYFP | CFP/YFP-A | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 435, 550 | 59033 ET Aqua/Gold FISH | ||||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 460, 580 | 460, 550 | 59022 ET eGFP/mCherry | GFP/DsRed-A | ||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 490, 580 | 59009 ET FITC/Cy3 | ||||
| pE-4000 (LLG Fit) | Fluorescence | Multiband | 490, 580 | 59010 ET Green/Red FISH #1 | ||||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | 365, 405, 490, 550, 635 | (365, 385, 405), 460, 580, 635 | 89400 ET DAPI/FITC/TRITC/Cy5 | LED-DA/FI/TR/Cy5-4X-B | ||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | (385, 405), 460, 550 | (365, 385, 405), 460, 580 | 89084v2 ET DAPI/Green/Orange FISH | LED-DA/FI/TX-3X-B | ||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | 405, 490, 550 | 385, 460, 550 | 69300 ET DAPI/FITC/TRITC | DA/FI/TR-3X-A | ||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | 385, 490, 580 | 89085 ET DAPI/Green/Red FISH | ||||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | 405, 490, 580 | 69302 ET DAPI/FITC/Texas Red | ||||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | 435, 490, 580 | 69308 ET eCFP/eYFP/mCherry | LED-CFP/YFP/mCherry-3X-A | |||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | 435, 490 | 59217 ET eCFP/eYFP | CFP/YFP-2X-A | |||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | 460, 580 | 490, 580 | 59222 ET eGFP/mCherry | FITC/TxRed-2X-B | ||
| pE-4000 (LLG Fit) | Fluorescence | Pinkel | 460, 550 | 59204 ET FITC/TRITC | GFP/DsRed-2X-A | |||
| pE-4000-L-SYS-ZZ: | pE-4000 Light Source with manual control pod, and power supply for LLG delivery |
| pE-4000-F-SYS-ZZ: | pE-4000 Light Source with manual control pod and power supply for fibre delivery |
| pE-4000-EB25D: | Rear mounting pE-Expansion Box for 25-way D-type to BNC connectivity |
| pE-4000-EFH-4: | Set of four Excitation Filter holders (25 mm dia.) |
| pE-6501: | USB-TTL Conversion Kit |
| pE-1906: | 1.5 m long, 3 mm diameter liquid light guide |
| pE-10400-YYY: | pE-Universal collimator for use with a single liquid light guide and customer specified adaptor. |
To specify microscope code (YYY), see Adaptors
To specify Power Cable (ZZ): 10 = Australia, 20 = Europe, 30 = UK, 40 = USA
The CoolLED pE Driver is first required for software integration, and can be downloaded here.
Contact CoolLED for further information.