Improving cell viability
Amongst the many benefits of LED illumination for widefield fluorescence microscopy is the potential for minimising the serious problem of photodamage in both fixed and live cells. Whether running a complete experiment or simply finding the region of interest prior to applying confocal microscopy, LED illumination systems are well suited to minimise both types of photodamage: phototoxicity and photobleaching.
Find out:
• Photodamage is not always obvious but can still skew results
• Mercury or metal halide lamps can easily damage samples
• LEDs reduce photodamage via enhanced control and TTL camera synchronisation
• Controlling widefield illumination is also important when setting up confocal experiments
Exposing biological samples to the excitation light levels typically used during a fluorescence microscopy experiment can have a detrimental effect, and countering photodamage is a crucial aspect of any fluorescence microscopy experiment.1 A variety of strategies exist, from specialised media to selecting bright, stable fluorophores.2 Even when employing non-widefield techniques such as light sheet microscopy to minimise sample exposure, locating the region of interest often relies on widefield fluorescence. For the majority of light microscope experiments, it is therefore vital to understand how the widefield light source itself can be used to minimise phototoxicity and photobleaching.
LED illumination systems are highly controllable compared to traditional mercury and metal halide lamps, and several key features can be used to optimise illumination and minimise photodamage.
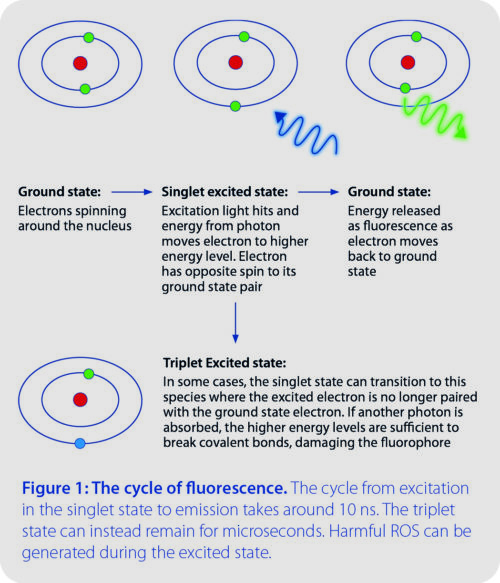
Photodamage explained:
Phototoxicity: This issue applies to live cell imaging only. Certain molecules within cells absorb photons and degrade when they react with oxygen, producing reactive oxygen species (ROS). Another source of ROS is the excited state of the fluorophores themselves (Figure 1). These ROS oxidize a range of other biomolecules, including DNA and proteins to cause irreparable cellular damage. The impact of phototoxicity can often be underestimated, since effects are not always immediately apparent. Cellular physiology can still be altered in subtle ways and can potentially lead to inaccurate conclusions, and the only way to truly detect phototoxicity is through experimental controls
Photobleaching: An issue for both fixed and live samples, signal is gradually lost from fluorophores due to atoms existing in the triplet state (Figure 1). Essentially, atoms in the triplet state can absorb enough energy to break covalent bonds and irreversibly damage the chemical structure – whilst also releasing ROS. The speed of photobleaching varies between fluorophores, but it can result in both data misinterpretation and time lapse experiments being cut short, limiting the observation of longer processes.
1. i) Less is more: use the gentlest illumination possible during sample observation
The most obvious and simple solution is to minimise the total light level at the sample plane. To add some perspective, a lamp at full power has been shown to reduce the fluorescence of Alexa Fluor 488 by half in just three seconds.3 Although this level of photodamage can occur whatever light source is in use, traditional lamps can only be modulated by inserting neutral density filters, which makes it challenging or impossible to reach the minimum possible irradiance where the sample is just visible.
LED illumination systems instead allow irradiance to be finely adjusted using sliders either on a control pod, graphical user interface or imaging software. Irradiance can therefore be minimised to the exact level where structures of interest are visible, yet illumination is gentle. It is also far better to start on the lowest irradiance setting and gradually increase this to the required level. When looking at the sample through the eyepiece, working in a dark room helps to further reduce this level as the dark opens your pupil such that a much lower amount of illumination on the sample will look sufficiently bright.
The irradiance of LED illumination systems can also be minimised to far lower levels than traditional lamps using ND filters, which is ideal for sensitive live samples. Another useful feature to look out for is individual channel control, limiting illumination only to the wavelengths required.
In some cases it can also be useful to determine the amount of light your sample is exposed to, and we recommend referring to our white paper explaining how to measure irradiance at the sample plane.4

1. ii) Balance exposure time with irradiance level
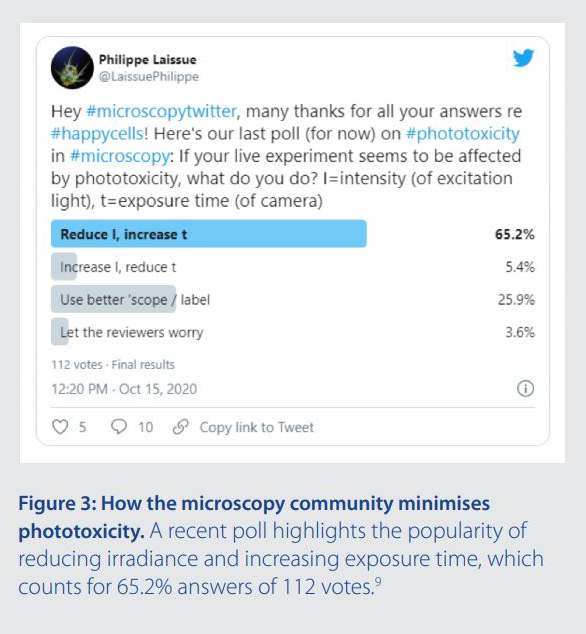
For image acquisition, lowering irradiance and increasing exposure time is a widely applied principle that can minimise photodamage. It has been evidenced in several research studies (albeit using lamp illumination).5-8 Anecdotally, it was also the most popular approach for minimising phototoxicity in a recent Twitter survey from Dr Philippe Laissue, (65.2% answers of 112 votes).9
One proposed explanation is that by exposing the sample to a lower number of photons at any one time point, the cell’s detoxification systems can cope with clearing the harmful ROS. However, this is still a theory and experimental conditions can differ in their illumination sensitivities. In cases where temporal resolution can be reduced, the ease of trialling irradiance versus exposure time using an LED illumination system makes this approach worth investigating for both fixed samples, and live cell imaging experiments which have controls to measure phototoxicity.10
2. Reduce ‘Illumination Overhead’
When acquiring images, especially in time lapse scenarios, another approach to minimise unnecessary sample exposure is making use of the electronic control options available with many LED illumination systems.
Traditional lamps use mechanical shutters, and even when triggered electronically still create a lag which can be hundreds of milliseconds. A descriptive term for this is ‘illumination overhead’, and is defined by the authors as: “the time fluorescent samples are exposed to incident light, but fluorescence emission is not being collected by the detector”.8
Electronic control of LED illumination systems drastically reduces illumination overhead. Synchronising the illumination system with the camera is possible using software control via a USB and achieves triggering speeds of up to 10 ms, depending on software and the PC operating system. Some LED illumination systems which feature a global TTL-in can also be synchronised directly to cameras including a TTL-out, and this can be as fast as <7 µs in the case of the CoolLED pE-800. This approach is especially valuable over the course of time lapse experiments and can result in improved cell viability and extended studies due to reduced photobleaching. These speeds have the added benefit of increasing temporal resolution, and for further information please see our in-depth review of hardware options which compares speed, contrast and budget requirements.11
3. Consider lower energy wavelengths
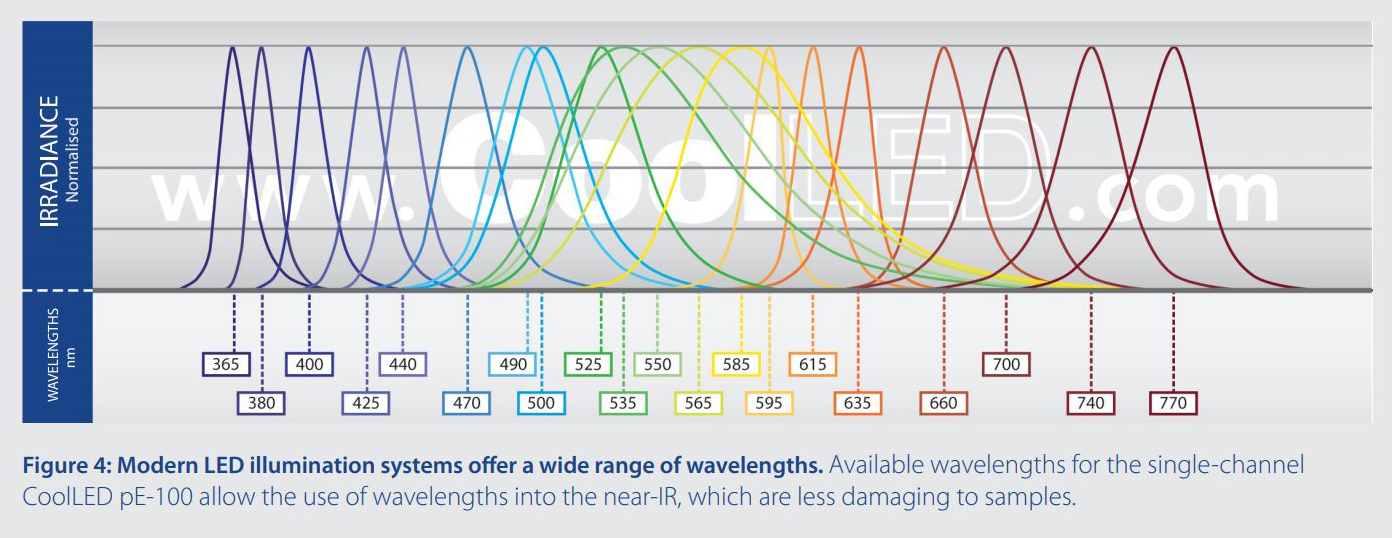
Traditional lamps tend to have a higher irradiance in the UV spectrum, which is more damaging towards samples, since these photons have higher energy and are more likely to be absorbed by biological materials. LED illumination systems offer far more choice in excitation wavelengths, for example 770 nm in the case of the CoolLED pE-4000 and pE-100 (Figure 4). These lower energy wavelengths are less damaging to live cells. As new fluorophores excited by longer wavelengths become available alongside the optical filters to match, re-assessing your LED, fluorophore and optical filter configuration may present another means of reducing sample photodamage.
4. Strobe illumination
Another interesting approach to reducing photobleaching is the use of strobe instead of constant illumination.2, 12, 13 Essentially, the theory is that constant illumination continues to create atoms in the fluorophore which exist in the triplet excited state (Figure 1). When light is instead delivered as a strobe with microsecond gaps, these triplet states have time to reset instead of damaging the fluorophore structure. Incredibly, this has been shown to achieve a 9-fold reduction
of EGFP photobleaching.13 Moreover, this approach is relatively simple to implement with the majority of modern, controllable illumination systems.
Conclusion
The first question we are often asked about CoolLED products usually relates to the power output, which is a relic from the days where LED illumination struggled to produce the output for fluorescence microscopy. The wealth of research and increased awareness in the area of photodamage has instead highlighted how “less is more”. In the vast majority of scenarios, modern LED illumination systems can deliver more than enough light to the sample – and this in itself can be problematic when not optimised. The first question for any light source manufacturer should instead focus on available control features.
Increasing numbers of labs and imaging facilities are upgrading from traditional lamps to LED illumination systems, making the features mentioned in this article available to scientists. Where it can be challenging to identify whether phototoxicity is impacting cell behaviour, it is important to err on the side of caution. Unfortunately, there is no ‘one size fits all’ approach and every experiment has different illumination sensitivities and requirements. For widefield fluorescence experiments, we therefore recommend taking the time to carefully optimise illumination settings or even investigate new fluorophore and optical filter combinations. Even when using widefield fluorescence briefly to set up confocal experiments, LED illumination systems can still help protect both fixed and live samples.
The topic of photodamage is broad and further advice can be found in many of the following references. Please also contact us if you have any questions.
References
1. Phototoxicity revisited. Nat Methods 15, 751 (2018). https://doi.org/10.1038/s41592-018-0170-4
2. Icha, J., Weber, M., Waters, J. C, & Norden, C. (2017). Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays, 39, . doi: 10.1002/bies.201700003
3. Jonkman, J., Brown, C.M., Wright, G.D. et al. (2020). Guidance for quantitative confocal microscopy. Nat Protoc. https://doi.org/10.1038/s41596-020-0307-7
4. CoolLED (2020). Measuring illumination intensity with accuracy and precision.
5. Koester HJ, Baur D, Uhl R, Hell SW. 1999. Ca2þ fluorescence imaging with pico- and femtosecond two-photon excitation: signal and photodamage. Biophys J 77: 2226–36.
6. Tinevez JY, Dragavon J, Baba-Aissa L, Roux P, et al. 2012. A quantitative method for measuring phototoxicity of a live cell imaging microscope. Methods Enzymol 506: 291–309.
7. Dixit R, Cyr R. 2003. Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. Plant J 36: 280–90.
8. Kiepas, A., et al. (2020). Optimizing live-cell fluorescence imaging conditions to minimize phototoxicity. Journal of cell science, 133(4), jcs242834. https://doi.org/10.1242/jcs.242834
9. Laissue Tweet
10. Laissue, P. P. et al. (2017). Assessing phototoxicity in live fluorescence imaging. Nature methods, 14(7), 657–661. https://doi.org/10.1038/nmeth.4344
11. CoolLED (2020). Capturing high-speed multi-labelled events with LED illumination systems.
12. Donnert, G., Eggeling, C., & Hell, S. W. (2007). Major signal increase in fluorescence microscopy through dark-state relaxation. Nature methods, 4(1), 81–86. https://doi.org/10.1038/nmeth986
13. Boudreau, C., Wee, T. L., Duh, Y. R., Couto, M. P., Ardakani, K. H., & Brown, C. M. (2016). Excitation Light Dose Engineering to Reduce Photo-bleaching and Photo-toxicity. Scientific reports, 6, 30892. https://doi.org/10.1038/srep30892










