Advancing Ex Vivo and In Vitro Optogenetics with LED Illumination
The development of LED illumination technology for life science research has significantly advanced fluorescence microscopy, but the speed and stability of LEDs also lends itself to optogenetics.
For optogenetic experiments performed under the microscope, a variety of LED Illumination Systems are available to suit different requirements – although choosing the ideal solution can be confusing.
We explain the different light source features that benefit optogenetics, and summarise the CoolLED Illumination Systems best suited to differing requirements.
Find out:
- How ex vivo and in vitro optogenetics experiments benefit from LED illumination
- What TTL and analogue LED control means for optogenetics
- Which LED illumination system is the best match for your requirements
Over the last decade, LEDs have become the go-to microscopy illumination technology for widefield fluorescence due to a wide range of factors – from convenience to cost and even sustainability. But microscopy is not the only technique that takes place under a microscope. Researchers are now finding use for fast, powerful and stable LED illumination in studies involving ex vivo optogenetics (using tissue slices), or in vitro optogenetics (using cell culture).
Why use LED illumination for optogenetics?
The solid-state nature of LED technology enables fast and stable illumination, while being more cost-effective, safer and longer lasting than lasers. The main reasons LEDs are a popular method of optogenetic stimulation include:
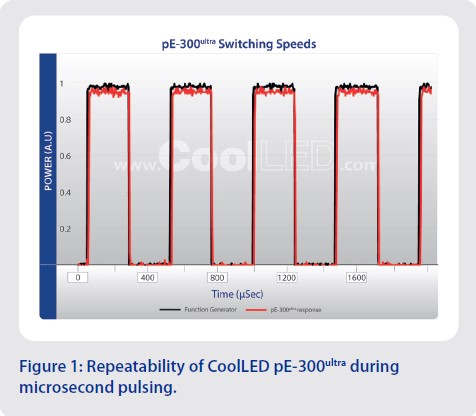
Repeatability: Even when pulsing at micro-second speeds, LED illumination is repeatable (Figure 1) and delivers precise photostimulation which is crucial when studying complex processes such as the intricacies of neuronal interactions.
Stability: LED irradiance remains stable over time, meaning the level of photostimulation remains precise between experiments performed even years apart.
Homogeneity: A high level of homogeneity across the field of view ensures every part of the sample receives the same level of photostimulation.
Speed: Fast light pulsing is crucial when working with fast cellular processes such as neuronal firing, and some LEDs can be triggered at speeds of under seven microseconds.
Control: In addition to standard software and manual control, some LED illumination systems can be synchronised with other equipment via TTL and analogue.
There are three main areas where microscope LED Illumination Systems are employed for optogenetics experiments:
• The optogenetic photostimulation itself is achieved via the microscope objective, which is often upstream of electrophysiology readings.
• Widefield fluorescence imaging can also be utilised as part of the experiment, for example to identify a sub-population of fluorescently labelled cells or perform calcium imaging measurements of the target cells.
• To provide morphological information, transmitted LED illumination systems can perform infrared DIC imaging.
For optogenetic photostimulation and widefield fluorescence, there is no ‘one size fits all’ for LED illumination systems, which vary significantly in their available features and budget. While this allows the researcher to choose the most suitable system for their requirements, it first requires an understanding of the two most important feature sets: wavelengths and control options.
LED Wavelengths and Channels
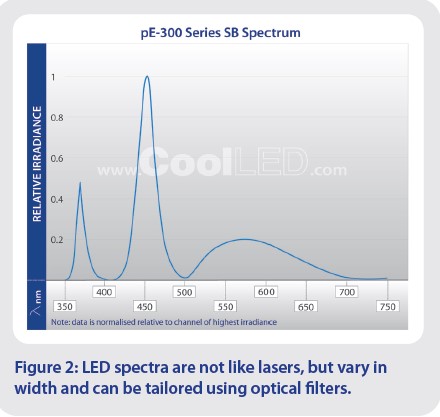
Before comparing LED illumination systems, it is first useful to appreciate the spectra of LEDs: they are not like lasers, and instead vary in spectral width. For example, the spectra of the pE-300 Series has a much wider green-yellow-red (GYR) LED, compared to the UV and blue LEDs (Figure 2). Using optical filters, they can therefore be tailored to a variety of opsins or fluorophores, even if the centre LED wavelength and peak excitation wavelengths are not a perfect match. To determine the compatibility of an LED with an opsin or fluorophore, we recommend using a tool such as FPbase to view spectral data of the illumination system and optical filter sets alongside the required opsins or fluorophores.1
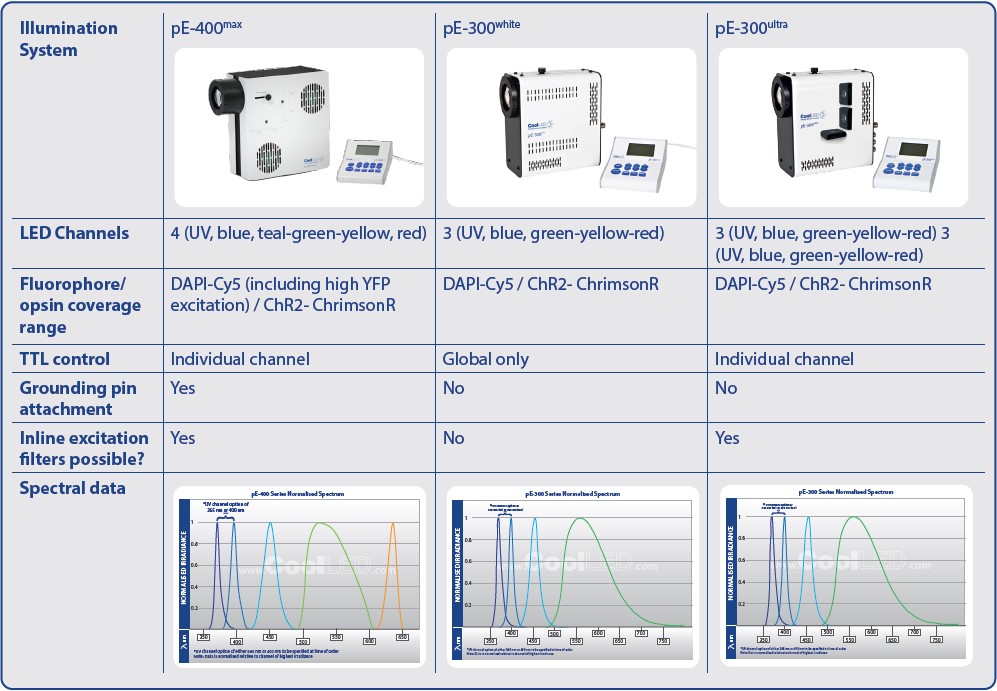
But it’s not just the LED complement to consider, and the number of individually controllable channels is equally important. The ability to control LED channels independently allows highly selective photostimulation, and for imaging, this reduces background from light outside the required excitation spectrum. The pE-300 Series provides an example to illustrate this difference between LED wavelengths and channel control. When comparing the pE-300lite to the pE-300ultra, the LED complement is identical, yet the LEDs of the pE-300lite are controlled globally (i.e., all three LEDs simultaneously) to produce a single channel of white light. Compare this to the pE-300ultra, where the LEDs are instead controlled individually as three separate channels.
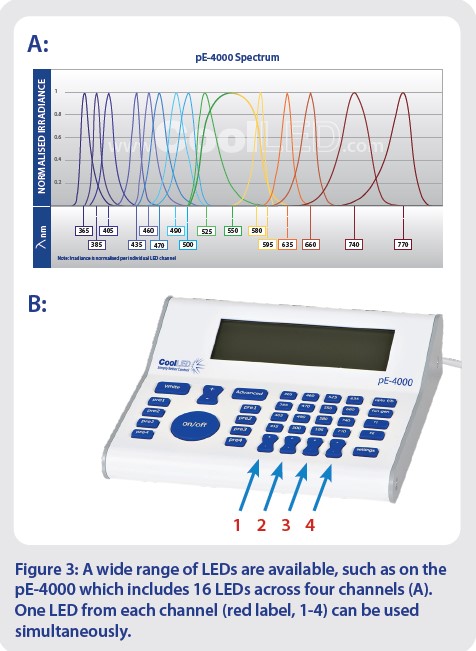
Many LEDs are now available to cover all popular opsins and fluorophores, and wavelengths within the longer reaches of the spectrum offer future-proofing as new red-shifted dyes emerge. For applications that demand greater control, Illumination Systems can include up to 16 LEDs (in the case of the pE-4000), spanning from the UV up to a peak wavelength of 770 nm in the near-IR region. These are separated into four channels, where one LED from each channel can be used simultaneously (Figure 3).
TTL & Analogue Control
Being solid-state, LEDs have a high level of spectral and temporal control – especially when compared to traditional lamps. For example, LED channels can be triggered individually, and the irradiance optimised from 0-100%, which is especially useful for protecting sensitive samples. These properties can be controlled through software via USB connectivity, which is available on most LED illumination systems, but this has its limitations in terms of speed and synchronisation. Transistor-Transistor Logic (TTL) input instead bypasses the latency of USB communication, allowing microsecond control of LED triggering.
The irradiance can also be modulated at high speeds where analogue input features, balancing brightness while minimising photodamage. In addition to speed-related benefits, this allows synchronisation of the illumination system with other peripheral components for tight control of aspects such as photostimulation, imaging and electrophysiology recordings.
As with all capabilities discussed so far, the exact level of control possible depends on the illumination system model.
Choosing an LED Illumination System
Advanced Control
For advanced applications, the choice sits between the CoolLED pE-4000 and pE-800.
The pE-4000 has the largest selection of wavelengths in the CoolLED product range, and is the only CoolLED Illumination System featuring TTL and analogue outputs (as well as inputs). The pE-800 instead features eight channels and industry-leading TTL triggering speeds of <7 μs. Therefore, the choice between these two Illumination Systems will mainly be determined by the priority of ultra-fast temporal resolution versus the need for synchronisation options.
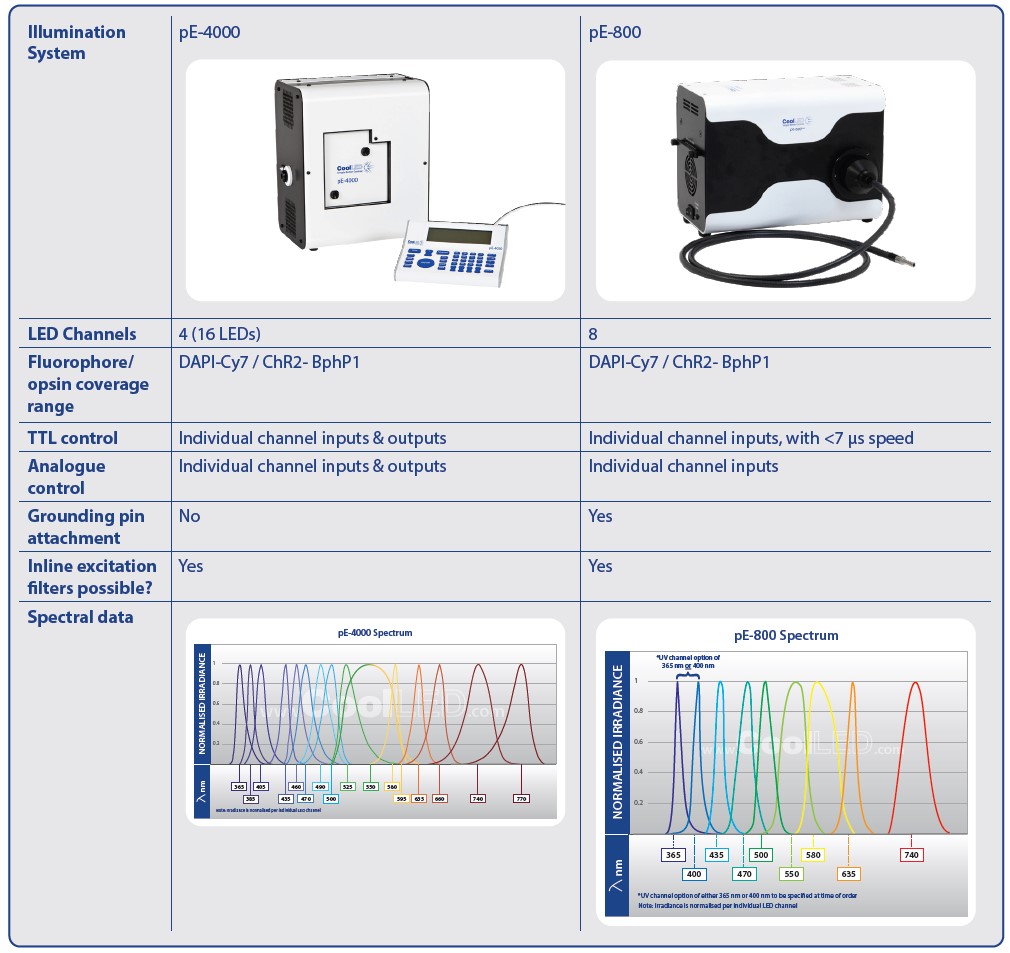
One additional feature is the facility to fit single-band excitation filters in front of each LED, which can be used to sharpen the photostimulation wavelength. For fluorescence microscopy with live samples, this can also enable high-speed, high-contrast imaging by using Pinkel filter sets.
Everyday Control
For optogenetics applications which do not demand the highest number of wavelengths or advanced synchronisation options, there are three Illumination Systems with suitable features. The pE-300ultra and pE-300white have three individually controllable channels, while the pE-400max has four individually controllable channels and significantly enhanced coverage for YFP. The pE-300white is the most cost-effective system of the three, but only has a global TTL input for controlling all LEDs together, instead of individual channel TTL triggering.
Cost-Effective Illumination
For stimulating a single opsin or fluorophore, a simple and cost-effective solution is the single wavelength pE-100, which has a choice of 20 different wavelengths ranging from 365 to 770 nm. It features a TTL input, and is also available with fibre-optic light delivery.

Plus Fura-2 Calcium Imaging
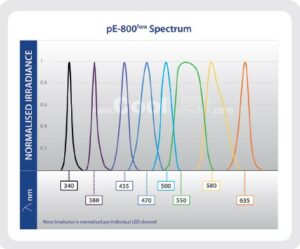
For neuroscience researchers wishing to maximise the utility of a single microscope, the eight channel pE-800fura Illumination System offers the same functionality as the pE-800 for advanced optogenetics, but includes a different LED complement, with two UV LEDs with peaks at 340 nm and 380 nm for fura-2 imaging. This also covers all popular opsins and fluorophores up to Cy5 and ChrimsonR, and LEDs are tailored using optical filters.

Transmitted Illumination
For IR-DIC, the pT-100 770 nm model is ideal and can be synchronised with the reflected light source with its TTL input.

Conclusion
Optogenetics is a powerful tool, especially when combined with the latest LED microscopy illumination technology. If you are considering upgrading an optogenetics light source to an LED illumination system, we strongly recommend spending the time to match the specifications to the application requirements to ensure experimental success – and we are always available to provide more information or answer any questions.