Graham D Wright1*, Sarah Zulkifli1, Tan Tong San2, Declan P Lunny2, John EA Common3 & E Birgitte Lane2
1IMB Microscopy Unit, 2Epithelial Biology Laboratory, 3Skin Barrier Laboratory, Institute of Medical Biology, A*STAR 8A Biomedical Grove, #06-06 Immunos, Singapore, 138648 *[email protected]
Epithelial cells and the sheets they produce, including our skin, are incredibly strong thanks to their internal keratin filament cytoskeleton networks. Mutations in the genes encoding keratin proteins lead to skin fragility disorders such as epidermolysis bullosa (EB). Individuals suffering from EB are known as “butterfly children” as their skin can blister with the slightest mechanical stress. DEBRA (www.debrasingapore.org) is a well-established charity supporting EB patients around the world. Research teams at the Institute of Medical Biology (IMB; www.a-star.edu.sg/imb) and the Skin Research Institute of Singapore (SRIS; www.astar.edu.sg/sris) within A*STAR are working to understand the biology behind the disease and the pathological consequences of keratin mutations.
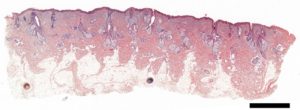
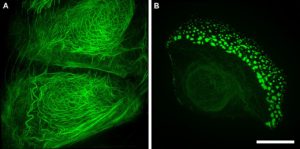
Research projects on keratins and EB rely heavily on techniques available within the IMB Microscopy Unit; from histological analysis (Fig. 1), through 3D and live-cell imaging (Fig. 2) to more recent developments such as superresolution microscopy (Fig. 3).The keratin filaments of the cytoskeleton play an important part in promoting, defining and maintaining epithelial differentiation and function. Networks of these keratin filaments link into anchorage points at cell-cell junctions providing the physical resilience for epithelial tissues to withstand normal wear and tear (Fig. 3A). In cells with the EB mutation these keratin networks are disrupted and aggregates form (Fig. 3B).
Figure 1. Digitised histological section (H&E staining) of human skin acquired on a MetaSystems Slide scanner. Scale bar = 2 mm
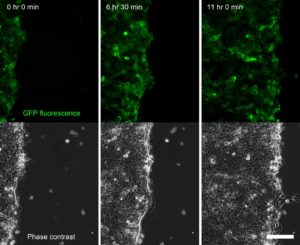
Figure 2. Time series of a confluent sheet of keratinocytes migrating during a live-cell imaging outgrowth assay, acquired on an Olympus IX-83 microscope equipped with a CoolLED pE-4000 lightsource (Fig. 4). Scale bar = 150 μm.
Figure 3. Structured illumination superresolution microscopy (3D-SIM) images of the GFP labelled keratin within (A) a wildtype and (B) a mutant keratinocyte cell. Acquired on a GE Healthcare DeltaVision OMX Blaze microscope. Scale bar = 10 μm.
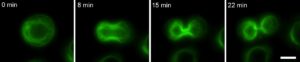
Our Olympus IX-83 live-cell microscope (Fig. 4), equipped with an LED light source (CoolLED) and stage top incubator (OKOLab) and focus maintenance system (Olympus ZDC) enables long term live-cell imaging experiments. These include scratch wound assays and outgrowth assays (Fig. 2) in which we can compare the behaviour of wildtype and mutant keratinocytes – the specialized epithelial cells of skin. Combined with powerful molecular biology techniques we are able to label the keratin filaments of interest with fluorescent proteins to observe and quantitate how they are organised and change during dynamic processes such as cell migration and cell division (Fig. 5).
Figure 4. The Olympus IX-83 microscope within the IMB Microscopy Unit is specifically set up for long term live-cell imaging experiments.
Figure 5. A keratinocyte undergoing mitosis in which the keratin is labelled with GFP.
Acquired on a Olympus IX-83 microscope – CoolLED pE-4000. Scale bar = 10 μm.
The range of microscopy techniques applied within this research project and the subsequent correlation of the results enables the research teams to understand how keratin proteins give rise to the mechanical strength of the skin and to elucidate the cell biology and dynamic processes that give rise to skin diseases. Through studying the mechanisms of keratin-related diseases we hope to enable better identification, diagnosis, and treatment to increase the quality of life for the patients.