
Malaghan Institute of Medical Research, Hugh Green Cytometry Centre
Allergies are characterized by the activation of a specific subset of effector CD4+ T cells, the T-helper type 2 (Th2) cells, that respond to harmless environmental allergens causing uncontrolled inflammation. The processes that drive the activation of Th2 cells in vivo are not completely understood. Dendritic cells (DCs) are key activators of CD4+T cells, producing molecules and mediators able to polarise naïve CD4+ T towards a Th2 phenotype.
To identify those signals, we performed bulk RNA sequencing of DCs isolated from skin draining lymph nodes, in mice exposed to allergens triggering (Connor et al, 2017). This analysis revealed that the chemokine Ccl17 was specifically upregulated by DCs in response to helminths and allergens known to skew immune responses towards a Th2 phenotype. We want to establish if Ccl17 is necessary for the priming of Th2 immune responses.
To investigate the transcriptional control of Ccl17 expression in DCs, we are performing a loss-of-function screen using the CRISPR-Cas9 technology on primary cultures of mouse DCs isolated from the bone marrow (BMDCs). Derived from a prokaryotic antiviral system, the CRISPR-Cas9 system consists of an endonuclease Cas9 and a single stranded guide RNA (gRNA). This gRNA contains a sequence that targets Cas9 to a specific genomic locus, within selected genes of interest. Co-expression of Cas9 and a gRNA creates a double-strand break in the target DNA, which can generate insertion or deletion leading to a loss-of-function mutation within the target gene.
The Cas9 protein is constitutively expressed in our mice and was combined with a Ccl17-eGFP reporter. The measure of the intensity of Ccl17-eGFP fluorescence reflects the level of expression of the chemokine in our BMDCs.
gRNAs sequences, associated with a mCherry reporter gene, are introduced in our BMDCs, using replication-defective lentiviral vectors. Successfully transduced BMDCs will express both Ccl17-eGFP and the mCherry reporter. If a gRNA induces a perturbation in a gene controlling the expression of Ccl17, we will observe a decrease in eGFP intensity.
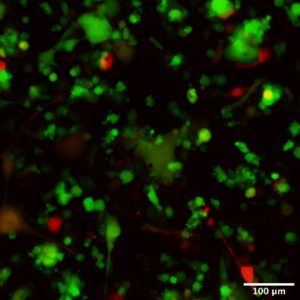
Our recently acquired CoolLED pE-300ultra fluorescence Illumination System has been a game changer in projects involving CRISPR-Cas9 screens. This new equipment is incredibly easy to use, and the 0-100% intensity option present in the control panel allows for visualization and recording of images with variable levels of fluorescence expression. Due to the possibility of switching off/on different wavelengths we can obtain a clean signal, essential for the detection of small variations of fluorescence intensity. Furthermore, the CoolLED pE-300ultra is LED based which allows us to move away from the mercury-based illumination system, towards a more “environmentally friendly” option, aligned with our commitment to sustainable practices in our activities.
Thank you to the authors for contributing this application note and choosing the charity Fair Earth Foundation to support. Find out how you can support your favourite registered charity and Share your Science!
