
Dr Martin Bootman, Reader in Biomedicine, Faculty of Science, Technology, Engineering & Mathematics
Analysing cellular signalling and sub-cellular processes are important aspects of cell biology research. For this we rely heavily on rapid, high resolution fluorescence measurement techniques – including Fura-2 ratiometric calcium imaging and the ability to stably image fluorophores in small cellular compartments.
1. Studying Mitochondrial Flickers
Mitochondrial membrane potential flickers are an interesting property of mitochondria. Although such membrane potential flickers have often been observed, how they are generated is not yet fully understood. Since these mitochondrial flickers are fast (rise time ~50 ms), successfully resolving this well-known phenomenon requires a microscope setup with high stability, good signal-to-noise, and adequate temporal resolution.1
To understand mitochondrial flickers in more detail, we tracked their temporal profile in HEK cells using our widefield fluorescence microscope setup (Zeiss Axio Observer 7 microscope with a Zeiss Axiocam 720) and a CoolLED pE-800fura Illumination System. Exciting the mitochondrial-specific tetramethylrhodamine ethyl ester (TMRE) indicator with the 550 nm LED, we visualised multiple mitochondria in the same cell (Video 1).
Visualising mitochondrial flickers.
In this video, you can see the flickering of the membrane potential of discrete mitochondria as rapid, reversible losses of TMRE fluorescence. You can also resolve the shape and movement of the mitochondria. HEK cells were loaded with the mitochondrial-specific TMRE indicator and excited using the 550 nm LED of the pE-800fura.
Analysing the TMRE fluorescence intensity within several small regions of interest that encompassed discrete mitochondria enabled us to track the flickers with good temporal resolution (Figure 1). The rapid downward deflections of TMRE fluorescence indicate the temporary drops in mitochondrial membrane potential that are known as flickers. To minimise photobleaching, we reduced the intensity of the Illumination System to 8% of the available LED light. In most cases, mitochondria flickered a couple of times before finally losing their fluorescence altogether.
Overall, we were impressed with the ability of the setup to successfully resolve the rapid initial phase of the flickers; several data points are evident during the initial rapid loss of TMRE fluorescence. Together, the images and fluorescence traces shown in Video 1 and Figure 1 indicate the stability and image quality achieved with the CoolLED pE-800fura.
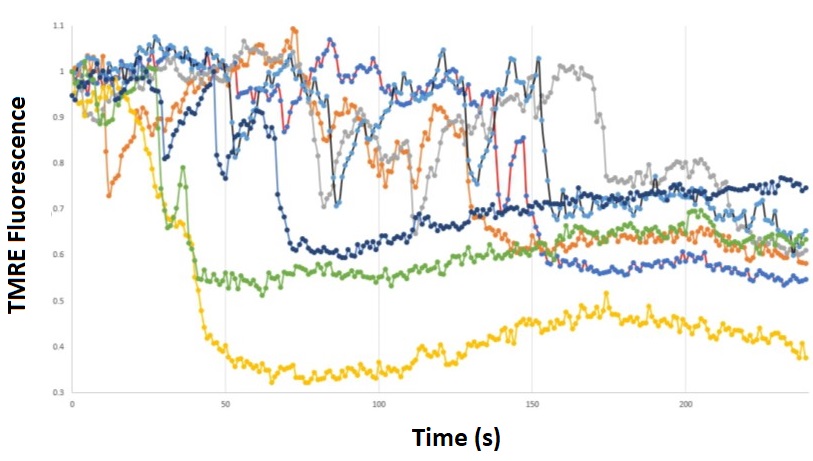
Figure 1: Tracking mitochondrial flickers.
HEK cells were loaded with the mitochondrial-specific TMRE indicator and excited at 550 nm. The TMRE fluorescence signal was obtained for seven discrete mitochondria from the same cell, and mitochondrial flickers can be identified by the rapid downward deflections in signal.
2. Fura-2 Ratiometric Calcium Imaging
Previously, we have performed ratiometric Fura-2 calcium measurements using the pE-340fura Illumination System, which includes 340 and 380 nm LEDs (in addition to a white light LED channel).
Figure 2 – Fura-2 calcium imaging with the pE-340fura
Cardiac myocytes loaded with Fura-2 using standard conditions (i.e. incubation with 2 micromolar Fura-2 acetoxymethyl ester for 30 minutes, followed by an additional 30 minutes for de-esterification. (Videos acquired by Martin Bootman and Katja Rietdorf).
The left-hand and right-hand videos show the Fura-2-loaded cells with 340 nm and 380 nm excitation, respectively.
The eight-channel pE-800fura also includes 340 and 380 nm LEDs which are identical to those of the pE-340fura. The major advantage of both Illumination Systems is lots of light with fast imaging, due to the fast channel switching – but of course the pE-340fura is mainly used for fast calcium measurements alone. If other wavelengths are required too, such as for multiplex fluorescence imaging, a multi-channel illumination system is more useful.
3. Standard multiplex fluorescence microscopy
The advantage of a multi-channel LED Illumination System is the versatility to suit different applications. In addition to mitochondrial analysis and calcium imaging, we can also use the pE-800fura for standard multiplex fluorescence microscopy.
However, as with all multi-wavelength LED Illumination Systems, the challenge is balancing image contrast with temporal resolution. For some applications where samples are not bright it might be necessary to slow down the image capture rate to allow a longer integration time. However, for sufficiently bright samples, it is possible to use multi-band filter sets that enable fast imaging. In either situation, the CoolLED Illumination Systems can be stably used for both extended single wavelength illumination or fast switching between wavelengths. Moreover, the LED light is consistent and stable, and the LEDs are super bright!
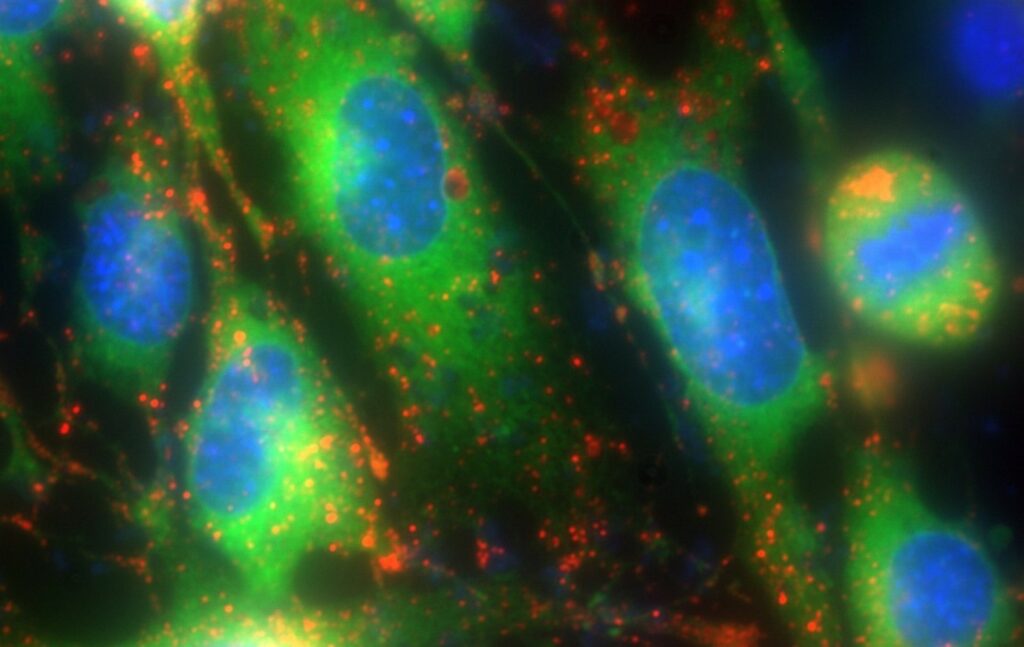
Figure 3 – Multiplex fluorescence microscopy.
Triple-labelled HEK cells showing the endoplasmic reticulum (ER-Tracker, green), lysosomes (LysoBrite, red), and cell nuclei (Hoescht, blue). Images were captured using a Zeiss Axio Observer 7 microscope with a Zeiss Axiocam 720 and CoolLED pE-800fura Illumination System with light intensity at ~5% for each channel.
References:
- Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002 Apr 2;21(7):1616-27. doi: 10.1093/emboj/21.7.1616. PMID: 11927546; PMCID: PMC125942.

About the CoolLED pE-800fura

For laboratories that rely on multiple applications including Fura-2 ratiometric calcium imaging, the pE-800fura offers several features:
- The ability for fast Fura-2 calcium imaging with rapid <7 µs switching between 340 nm and 380 nm, with no dichroic mirror change.
- Fast high-contrast imaging that is only slowed by the need to switch in a dichroic for other colours required.
- Eight LED channels allowing customisable colour choices with standard multi-colour imaging (where the trade-off between speed and contrast depends on optical filters).
- High power and stability.
