Using Optogenetics to analyse neuronal circuits.
Dr Max Anstotz – University Medical Center Hamburg-Eppendorf, Institute of Neuroanatomy
Analysing neuronal circuits is crucial for the understanding of basic and complex cortical functions. Over the last decades, many methods have emerged for the analysis of connections between neurons. Patch-Clamp electrophysiology (specifically paired-recordings), combined with post-hoc morphological analysis, is commonly used to study the local connection between neurons. The examination of a synaptically coupled neuron pair allows detailed insights into their physiology and function. Performing paired-recordings, a pre-synaptic (signal- sending) neuron is stimulated allowing the recording of evoked signals in a connected post-synaptic (signal-receiving) neuron.
Whereas this could be seen as the “gold-standard”, the low probability (~1-3% per tested pair) of finding a connection makes it extremely time-consuming. This low probability can be explained by the network integrity after acute brain slicing and/or sparse inter-connectivity in certain brain areas. Furthermore, if a connection between specific neuron types is unclear, valid data are hard to gain and take up many valuable days of experimenting.
During the last years, a new experimental approach has become popular. By expressing light-sensitive proteins in neurons, the user is able to modulate the membrane potential and generate (depending on the expressed protein) either excitation or inhibition via light.
A very common light-sensitive ion channel is channelrhodopsin 2 (ChR2). With its peak excitation at blue light (~470nm), pulses in this spectrum allow a sodium influx in cells. This mechanism is combined with the promotor specific expression using genetic models such as the Cre/loxP-System.
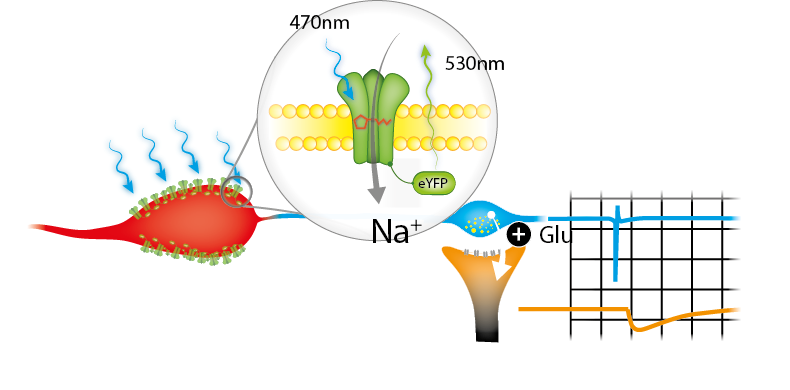
Exposing a certain area of genetically modified brain-tissue with blue light generates action potentials in the desired neurons. This results in a neurotransmitter release at pre-synaptic terminals of specific neurons. Putative post-synaptic currents/potentials can then be recorded in connected neurons. The specificity of this connection can further be validated by adding pharmacological blockers (e.g. NBQX).

To upgrade our common patch-clamp electrophysiology system and make it capable of performing optogenetic experiments, the fluorescence light source was altered . We needed an easy to install, triggerable, intensity- modulated light source. An LED is by far the easiest and cheapest solution. Furthermore, small latencies between triggered light pulses are crucial for the analysis of the kinetics of measurements. We used the CoolLED pE-300ultra, which meets these requirements. Another great advantage of this product lies in its versatility for use in upcoming experiments covering different wavelengths. Its flexibility allows the performance of very complex experimental protocols.
We have taken advantage of a transgenic mouse model expressing ChR2 under a desired promoter (using a Cre-loxP system). Hence, a specific neuron class (Neuron A) can be stimulated by light.
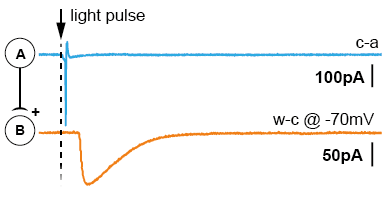
After acute brain slicing, preparations were made for common patch-clamp electrophysiology. The acute brain slice was placed in the microscope bath-chamber. We patched a putatively connected post-synaptic neuron (Neuron-B) in whole-cell configuration. By giving short light pulses (1-3ms) to the entire slice/view-field we were able to generate action potentials in a large cluster of pre-synaptic neurons (Neuron-A). Post-synaptic currents generated by the transmitter release were recorded and analyzed. The results suggest the potential that Neuron-class-A projects to Neuron-class-B. The short latencies of the triggered light-pulse further allowed the examination of di-synaptic connections (Neuron-A to Neuron-B to Neuron-C).
There are two main advantages using this approach. First, the pre-synaptic neuron doesn’t need to be stimulated via patch-clamp electrophysiology, saving time and resources. Secondly, light pulses activate several pre-synaptic neurons, massively increasing the probability of finding a connection (up to 90% per tested post-synaptic neuron).
Neither specific mono-synaptic characteristics nor exact contact sites of the synapses can be evaluated, but with these preliminary data the time-consuming approach of paired-recordings is then evaluable as an adjacent experiment.
A second advantage of the system, beside optogenetics, is that the LED can be connected to the Camera Trigger. We simply connected the trigger output to the global shutter of the CoolLED pE-300ultra. This replaces an Illumination-Shutter and greatly reduces photo-toxicity, bleaching and is much more convenient. Turning on the live view function in the cameras acquisition software then automatically switches on the camera.

